Bone Sarcoma Research Laboratory
In the laboratory, we aim to better understand the biology of bone sarcoma to identify novel therapeutic strategies against these aggressive cancers.
Aggressive primary bone cancers such as osteosarcoma and Ewing sarcoma typically occur around the age of adolescence. These patients are currently treated with polychemotherapy, surgery, and radiotherapy. Over the past decades, clinical trials combining and optimizing these treatments allowed to drastically increase the survival rate for these patients, but tend to reach their limits to achieve additional therapeutic improvements. Furthermore, therapeutic options for patients with recurrent and/or metastatic diseases are still limited and the long-term consequences of current treatments are not negligible. New therapeutic approaches to treat these patients are therefore an urgent need.
The development of "next-generation" sequencing approaches has enabled the discovery of recurrent genetic alterations in cancers, including in bone sarcoma. Drugs targeting the consequences of these genetic lesions have been and continue to be extensively developed and have revolutionized cancer treatment. Unfortunately, most recurrent alterations identified in these primary bone cancers belong to the currently "undruggable" category. To overcome this aspect, our laboratory aims at using cutting-edge cellular and molecular approaches to identify alternative approaches to treat these patients and to decipher how cancer cells metastasize and resist treatments. For this, three projects are currently conducted in the laboratory:
Exploring synthetic lethal mechanisms in bone sarcoma as novel therapeutic opportunities
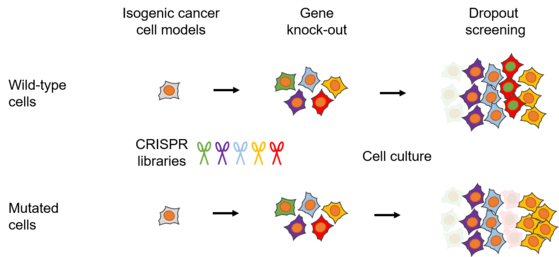
Since most recurrent genetic events in Ewing sarcoma belong to the rather undruggable category or conduct to loss of function events, we aim at identifying alternative targetable genes/pathways to identify novel therapeutic opportunities for these mutated cancers. For this, we are using genome-wide and targeted pooled CRISPR screening approaches to identify candidate genes that preferentially kill mutated cells (highlighted below in red) but not wild-type cells (concept of synthetic lethality).
Preventing and exploring metastasis mechanisms in bone sarcoma
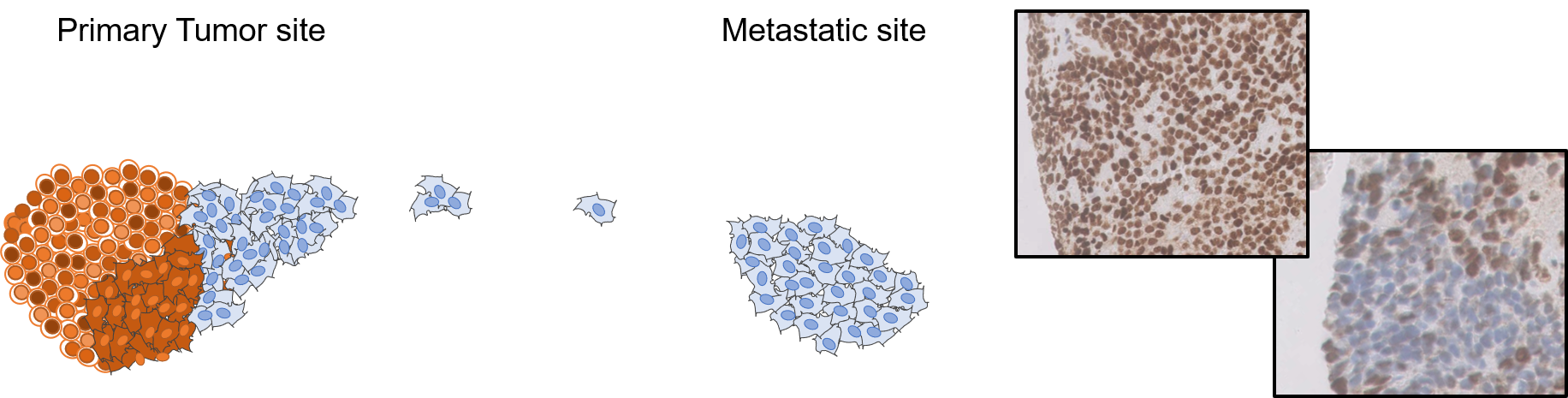
Ewing sarcoma cells display a strong capability to metastasize. Nearly a quarter of patients present with metastasis at the time of diagnosis, which is a major poor prognosis factor for these patients. Mechanisms leading to metastasis in Ewing sarcoma are however only partially understood and multiple. The goal of this project is to better understand the origin and the spatiotemporal evolution conducting these cancers cells to spread and resist current treatments.
Patient-derived bone sarcoma modeling and drug profiling
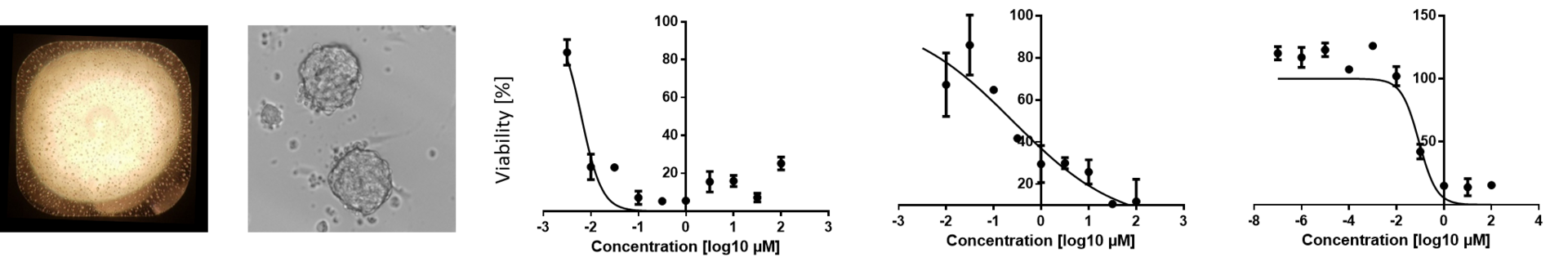
Providing faithful cellular models for bone sarcoma is of prime importance to predict treatment response in patients and to better understand their biology. In this collaborative project conducted at the University of Zurich (Clinical Research Priority Program), we aim to develop next-generation drug profiling platforms for clinical application in cancer, to perform drug response profiling to guide treatment in trials, and to gain mechanistic insights into these cancers.
Selected Publications
- Surdez D, Zaidi S, Grossetête S et al. STAG2 mutations alter CTCF-anchored loop extrusion, cis-regulatory interactions and impact EWSR1-FLI1 activity in Ewing sarcoma. Cancer Cell. 2021;28:1535-6108(21)00208-7.
- Solé A, Grossetête-Lalami S, Surdez D et al. Unraveling Ewing sarcoma tumorigenesis originating from patient-derived Mesenchymal Stem Cells. Cancer Research. 2021.
- Surdez D, Landuzzi L, Scotlandi K, Manara MC. Ewing Sarcoma PDX Models. Methods Mol Biol. 2021;2226:223-242.
- Grünewald TGP, Cidre-Aranaz F, Surdez D et al. Ewing sarcoma. Nat Rev Dis Primers. 2018;4(1):5.
- Tirode F, Surdez D, Ma X et al. Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discovery. 2014;4(11):1342-5.
Contact
We look forward to hearing from you.

Prof. Didier Surdez
Head of Tumor Research
+41 44 510 74 22
Email